Welcome to the fascinating world of tissue engineering where science and imagination blend seamlessly. Our cutting-edge techniques pave the way for innovative solutions that harness the power of cellular engineering. From regenerating damaged tissues to creating customized, life-changing medical implants, our team of talented researchers and scientists are revolutionizing the field. With meticulous precision and a passion for excellence, we are shaping the future of healthcare, one cell at a time. Embark on a journey of endless possibilities with tissue engineering and unlock the potential of tomorrow, today.
Keywords: Intervertebral disc tissue engineering, Interbody Cage, Spine Tissue engineering.
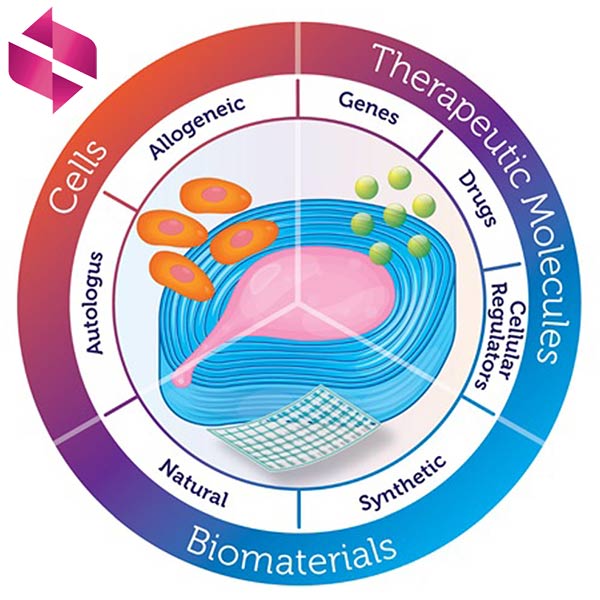
Intervertebral Tissue Enginnering
World of tissue engineering, where breakthrough discoveries are redefining the boundaries of medical science. At the forefront of this exciting field, we focus on three essential elements: the dynamic cells that tirelessly create and remodel neotissue, the influential soluble factors like growth factors that regulate cell function, and the scaffold materials that provide an optimal environment for tissue repair. Unlike traditional views, we see the potential of scaffolds as more than just substrates for cell attachment and culture; instead, we maximize their role in enhancing cellular functions crucial for tissue regeneration. Join us on this extraordinary journey of limitless possibilities, as we revolutionize healthcare, one cell at a time.
In the ever-evolving realm of mechanobiological research, scientists are delving into the intricate ways that cells perceive and respond to their surrounding environment. While past studies have primarily focused on materials that mimic the macroscopic scale of cells, there is now a growing interest in designing finer-scale environments that can modulate cellular function. By understanding how cells interpret mechanical stimuli, we can uncover insights into critical processes like cell migration, proliferation, secretion, and differentiation. Recent investigations have revealed that the formation of focal adhesion, a complex involved in cell adhesion and migration, is regulated by factors such as the distribution of extracellular matrix (ECM) ligands and substrate rigidity. However, the biological environment is multifaceted and still holds many unresolved mysteries, including interactions between different cell types and the ever-changing properties of materials. To shed light on these cellular regulatory processes, researchers have utilized materials and models that possess structural similarities to living tissues. Notably, a recent study showcased a breakthrough in the mechanotransduction signaling pathway. By administering a small-molecule inhibitor to a porcine model of autologous segmental skin grafts, the study demonstrated a shift towards anti-inflammation, differentiation of fibroblasts, reduced contractures, alleviated scar formation, and reconstructed collagen structure.
In a simple and preliminary perspective, the use of regenerative medicine techniques may seem very promising, and indeed it may be promising in the future, but currently, a structure that fully supports the fundamental conditions of regenerative medicine and tissue engineering is not commercially available; one contentious condition is the use of scaffolds that can provide both initial support and support during reconstruction and repair from the damaged site. Clearly, the explanation of this phenomenon is such that tissue reconstruction occurs over a specific period of time at a certain rate; having a scaffold that can provide both initial mechanical support, biocompatibility and other key properties need for intervertebral disc during the specified period of tissue reconstruction and be biologically degraded at a controlled rate (ideally the rate which is equal to the functional tissue reconstruction rate at the injury site) is practically challenging; perhaps feasible on a laboratory scale currently, but commercially having such structures is difficult. This mentioned difficulty can be examined from several perspectives: 1) optimizing biocompatible materials capable of biodegradation over a specified period of time, 2) depending on the type of materials, the construction method of these structures becomes important, 3) sterilization of these structures depending on their form may be challenging, and 4) maintaining these structures may be challenging depending on the previous factors, and if these products do not have an off-the-shelf solution, they cannot have a large market commercially.
Despite all the factors mentioned, however, utilizing tissue engineering approaches is not discouraging at all and will not be; because incorporating tissue engineering principles into improving current products can lead to the creation of products that can be completely developed and produced in accordance with tissue engineering principles and commercially available.
In recent times, interbody fusion cages have become crucial in surgery for conditions like disc protrusion, disc herniation and spondylolisthesis. However, conventional cages often fail to deliver satisfactory outcomes due to their flawed design, inadequate material compatibility, and limited osteogenesis capacity, thus restricting their utility. Currently, there are three methods to enhance fusion effectiveness: Firstly, optimizing the cage design to promote bone growth. Secondly, utilizing cages crafted from diverse materials to cater to varying fusion requirements. Lastly, employing post-processing techniques, such as coating the cage and incorporating bioactive materials, to foster appropriate osseointegration and create an optimal biological environment for bone tissue, thereby enhancing fusion outcomes.
References
For more information about this topic, you can use these articles:
1. Hama R, Reinhardt JW, Ulziibayar A, Watanabe T, Kelly J, Shinoka T. Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration. Biomimetics. 2023; 8(1):130. https://doi.org/10.3390/biomimetics8010130
2. Zhang H, Wang Z, Wang Y, Li Z, Chao B, Liu S, Luo W, Jiao J and Wu M. Biomaterials for Interbody Fusion in Bone Tissue Engineering. Frontiers in Bioengineering and Biotechnology. 2022; 10:900992. https://doi.org/10.3389/fbioe.2022.900992
