
Throughout human history, physical injuries have been an inseparable part of everyday life, and even genetic defects have imposed limitations on humans from the past to the present. Since then, humans have always sought ways to overcome these limitations.
Initially, humans sought to aid in wound healing and self-surgery using natural textiles. Over time, with the advancement of science, using bandages for fractures became common, representing one form of temporary biomaterials for restoring function or supporting damaged tissue.
A biomaterial is a substance that, when exposed to living tissue, should avoid triggering unwanted immune responses and be capable of restoring the tissue or at least its function. In this article, we intend to start with the definition of biomaterials and introduce different generations of biomaterials.
What is “Biomaterial”?
The general definition of a biomaterial is what was presented in the introduction section of this article, but the definition of biomaterial according to what the National Institute of Health (NIH) states as follows: “any natural or synthetic substance or combination of substances, other than drugs, which can be used to augment or partially or totally replace any tissue, organ or function of the body, in order to maintain or improve quality of life of individual”.
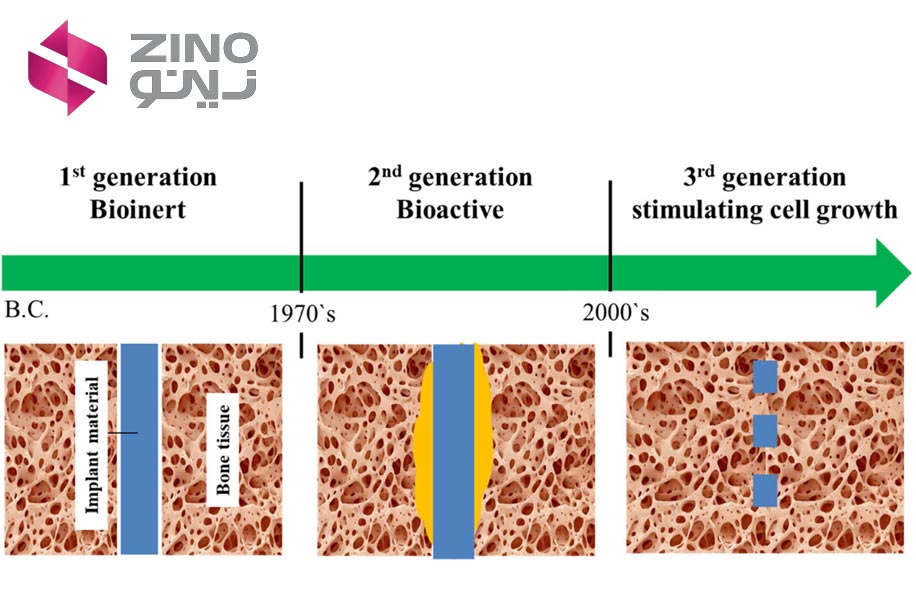
First Generation of biomaterial
(Bioinert Age)
In the 1960s and 1970s, a first wave of materials was specifically designed for internal use within the human body, laying the groundwork for the biomaterials field. These materials gave rise to prostheses, devices crafted from biomaterials.
Professor Bill Bonfield was among the pioneers who recognized the importance of comprehending the mechanical traits of tissues, particularly bones, to ensure dependable skeletal prostheses. His work marked an early effort to grasp how biomaterials interact with living tissues. Initially, the focus of biomaterials was to attain a suitable blend of physical properties mimicking those of replaced tissues while evoking minimal toxic reactions in the host.
By 1980, over 50 implanted prostheses, made from 40 different materials, were in clinical use. Globally, more than three million prosthetic parts are being implanted annually. The majority of the materials of the 1980 article were characterized by their biological inertness. Most materials used in implants were single-phase materials, often adaptations of existing commercial materials with enhanced purity levels to prevent the release of toxic by-products and reduce corrosion.
The fundamental aim driving the majority of biomaterials advancement from the 1960s to the early 1980s was to minimize the biological reaction to foreign bodies. Remarkably, this engineering principle remains relevant even four decades later. Countless individuals have experienced a significant improvement in their quality of life for up to 25 years or more through the use of implants crafted from ‘bioinert’ biomaterials. These biomaterials typically result in the formation of a thin, acellular fibrous capsule at the interface with tissues, exhibiting minimal, if any, adhesion between the implant and host tissue.
Nevertheless, Professor Bonfield recognized the necessity for a new generation of biomaterials. His research group embarked on exploring the concept of designing biocomposite materials in the 1980s that closely mirrored the mechanical properties of host bone. These composites aimed to mitigate, or even eradicate, stress shielding and bone resorption at the prosthesis-bone interface caused by a mismatch in elastic modulus. Devices with higher moduli bear the majority of the mechanical load, leading to gradual bone resorption, strength deterioration, and eventual failure.
The biocomposites developed by Professor Bonfield’s laboratory introduced the innovative concept of bioactivity, alongside the design principle of tailoring two phases to achieve composite properties closer to those of living bone. This pioneering work resulted in a groundbreaking composite material, known as Hapex, comprising a polymeric matrix of polyethylene and a dispersed bioactive phase of hydroxyapatite (HA) particles. Hapex marked the first successful biocomposite material specially engineered for medical devices.
Continuing his pioneering contributions, Professor Bonfield developed a novel type of bioactive material, Si-substituted HA, which has proven successful as a bone graft material. His extensive understanding and interdisciplinary contributions enabled him to co-found and edit the highly impactful Journal of the Royal Society Interface.
Second Generation of biomaterial (Bioactive/Bioresorbable Age)
Progress in comprehending biological mechanisms has enhanced our grasp of how biological entities interact with surfaces made of biomaterials. The evolution in second-generation biomedical materials research shifted focus from inert materials to those that actively engage and merge with the biological milieu.
By the mid-1980s, bioactive materials had begun to be utilized clinically in various orthopedic and dental scenarios. Different formulations of bioactive glasses, ceramics, glass-ceramics, and composites were undergoing clinical testing. Synthetic hydroxyapatite (HA) ceramics were being regularly employed as porous implants, powders, and coatings on metallic prostheses to facilitate bioactive bonding. The incorporation of minimally soluble HA coatings prompted a tissue response known as osteoconduction, where bone growth occurred alongside the coating, forming a robust interface. The mechanically resilient and durable bioactive A/W glass-ceramic, developed at Kyoto University, was deployed for replacing vertebrae in patients with spinal tumors. In 1998, a feature article by the American Ceramic Society highlighted the swift adoption of first- and second-generation bioceramics in clinical settings.
During this era, another class of second-generation resorbable biomaterials gained clinical significance. The aim of resorbable biomaterials was encapsulated in a statement from 1980, which outlined the objective as follows:
An alternative approach to modulating the interaction between biomaterials and tissue involves regulated chemical degradation, known as resorption of the material. The resorption of biomaterials presents an ideal solution to the challenge of the biomaterial-tissue interface since the foreign material is eventually replaced by regenerated tissues. Ideally, over time, there should be no noticeable distinction between the site of implantation and the surrounding host tissue.
Third Generation of biomaterial
(Bioresorbable and Bioactive Convergence)
The third generation of biomaterials were materials that realized tissue engineering principles. These biomaterials are materials with specific and engineered structures that can influence cellular behavior at the molecular level to facilitate tissue regeneration effectively. The third generation of biomaterials is essentially an advancement of the second generation. In the second generation, after complete tissue reconstruction in the joint area, the boundary between the implant and the regenerated tissue was not clearly defined. In the third generation of biomaterials, in addition to this issue, the structure of the implant gradually deteriorates as the damaged tissue is regenerated, and functional tissue takes its place, so that after complete tissue regeneration, there is no structure other than body tissue in that area.
The materials used with the approach of the third generation of biomaterials must simultaneously possess bioactive and bioabsorbable material properties, and tissue reconstruction activity at the molecular level should be stimulated by the degradation of their structure and absorption of their components, leading to the regeneration of relevant genes.
Forth Generation of biomaterial
With the advancement of cellular and molecular biology, it has been understood that the origin of most cellular behaviors is dependent on the activity of pumps and ion channels. Therefore, some researchers believe that the fourth generation of biomaterials should be such that it can manipulate and regulate bioelectric signals for tissue regeneration, as well as monitor cellular behaviors. Evaluating bioelectric behaviors can provide detailed information about the host tissue’s response to implants and thus assess tissue improvement processes.
References
For more information about this topic, you can use these references:
1. Farag, M.M., Recent trends on biomaterials for tissue regeneration applications: review, J Mater Sci (2023) 58:527–558. https://doi.org/10.1007/s10853-022-08102-x
2. Bahrami, H., and Gholipour-Kanani, A. Nanofibers in regenerative medicine and tissue engineering, Chapter 1, introduction and history, 2017.
3. Ning, C., Zhou, L., Tan, G., Fourth-generation biomedical materials, Materials Today, Volume 19, Issue 1, 2016, Pages 2-3. https://doi.org/10.1016/j.mattod.2015.11.005
4. Hench, L.L., Thompson, I., Twenty-first century challenges for biomaterials, J R Soc Interface. 2010 Aug 6;7 Suppl 4(Suppl 4):S379-91. https://doi.org/ 10.1098/rsif.2010.0151.
